

转自:康龙化成夜夜撸改成什么
RingContractionofSaturatedCyclicAminesandRearrangementofAcyclicAminesThroughTheirCorrespondingHydroxylamines
YiPeng[a],GuoqiangWang[b],*HendrikF.T.Klare[a],andMartinOestreich[a]*
[a]InstitutfürChemie,TechnischeUniversitätBerlin,Strassedes17.Juni115,10623Berlin(Germany);
[b]InstituteofTheoreticalandComputationalChemistry,SchoolofChemistryandChemicalEngineering,NanjingUniversity,Nanjing,210023,P.R.China
—Angew.Chem.Int.Ed.,2024,63,e202410483
RecommendedbyRuiJin_MC5

KEYWORDS:skeletalediting(反馈类型),ringcontraction(反馈类型),rearrangement(反馈类型),C(sp3)–N(成键类型),B(C6F5)3(催化剂),hydrosilane(试剂),hydroxylamine(原料),amine(居品),scaffoldhopping(其他),targetedcarbonatomdeletion(其他)
ABSTRACT:Comparedtomodificationsatthemolecularperiphery,skeletaladjustmentspresentgreaterchallenges.Withinthiscontext,skeletalrearrangementtechnologystandsoutforitssignificantadvantagesinrapidlyachievingstructuraldiversity.Yet,thedevelopmentofthistechnologyforringcontractionofsaturatedcyclicaminesremainsexceedinglyrare.Whilemostexistingmethodsrelyonspecificsubstitutionpatternstoachieveringcontraction,thereisapersistentdemandforamoregeneralstrategyforsubstitution-freecyclicamines.Toaddressthisissue,wereportaB(C6F5)3-catalyzedskeletalrearrangementofhydroxylamineswithhydrosilanes.Thismethodology,whencombinedwiththeN-hydroxylationofamines,enablestheregioselectiveringcontractionofcyclicaminesandprovesequallyeffectiveforrapidreorganizationofacyclicamineskeletons.Bythis,thedirectscaffoldhoppingofdrugmoleculesandthestrategicdeletionofcarbonatomsareachievedinamildmanner.Basedonmechanisticexperimentsanddensityfunctionaltheorycalculations,apossiblemechanismforthisprocessisproposed.
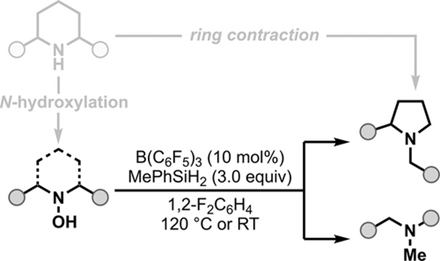
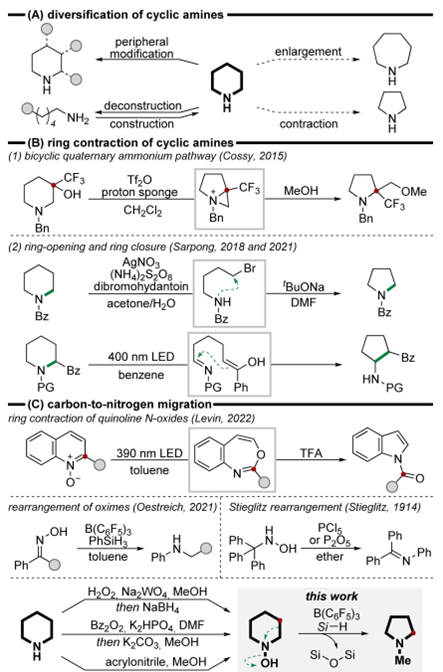
Reactiondesignanddevelopment
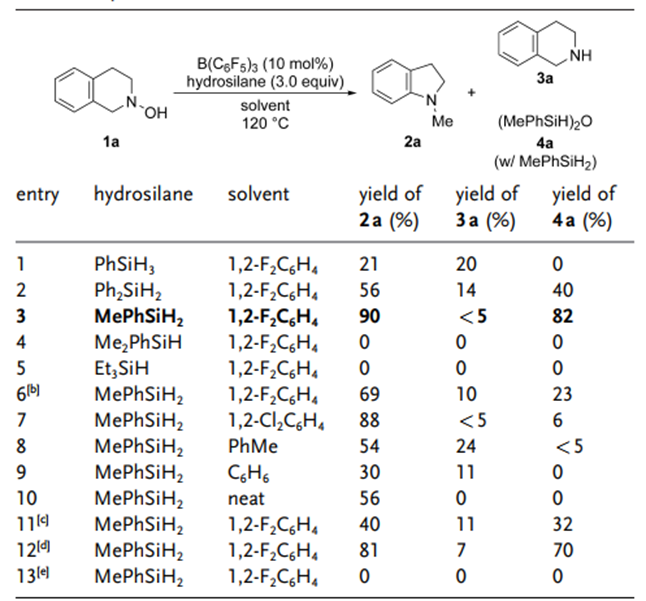
Optimizationofthereactionconditions
Scopeofsaturatedcyclicaminesintheringcontractionandapplicationsofthering-contractionstrategy
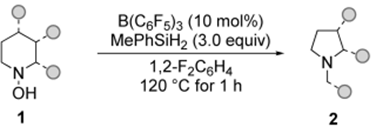
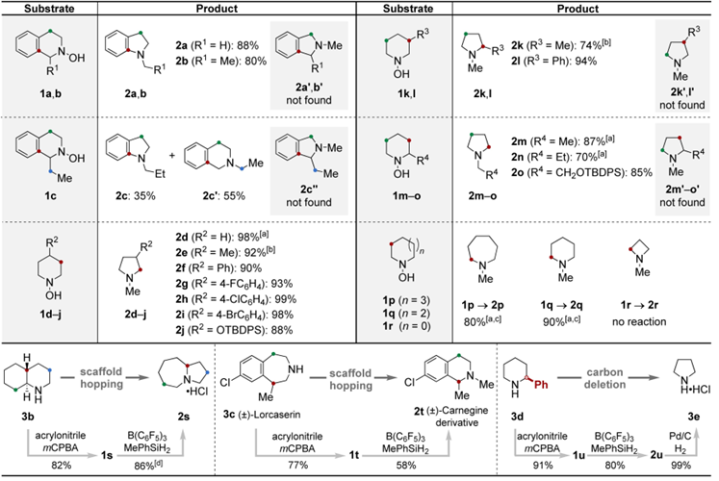
Scopeofacyclicsubstratesinskeletalreorganization
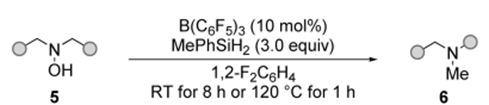
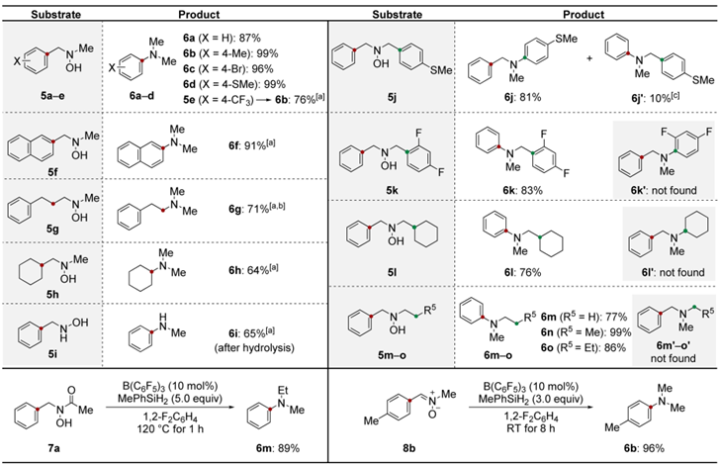
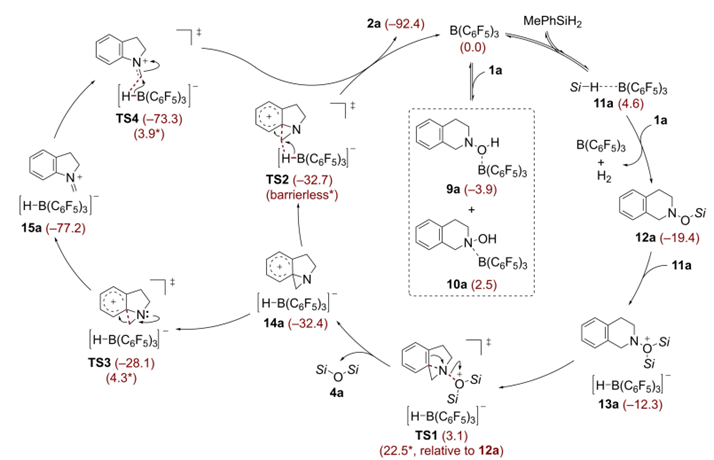
Reactionpathwaysfortheringcontractionofhydroxylamine1awithMePhSiH2
SummaryandComments
Insummary,OestreichandWangetal.havedevelopedaB(C6F5)3-catalyzedskeletalrearrangementofhydroxylamineswithhydrosilanes,overcomingthelimitationsofthetraditionaluncatalyzedStieglitzrearrangement.Thisadvancement,whenintegratedwithefficientN-hydroxylationtechniquesforamines,enablestherapidreorganizationofamineskeletons,particularlyshowcasinghighlyregioselectiveringcontractionofcyclicamines.Incontrasttopreviousmethodsthatrequiredspecificsubstitutionpatternsonthering,ourrearrangementstrategydemonstratesenhancedflexibility,accommodatingawidearrayofcyclicamines.Thepotentialofourstrategyforscaffoldhoppingandtargetedcarbonatomdeletionhasbeensubstantiated.DFTcalculationsshowthattheoverallreactionisanexergonicprocess.Althoughtwopotentialpathwaysfortheringopeningoftheimplicatedphenoniumionarekineticallyviable,thebarrierlesshydridetransferfromtheborohydridetothemethylenegroupwithinthephenoniumionappearstobethepreferredpathway,comparedtothedirectringopeningofthephenoniumionwithahigherenergybarrier.
柏林工业大学MartinOestreich与南京大学GuoqiangWand课题组共同报说念了一类羟胺在B(C6F5)3催化以及MePhSiH2的作用下发生的分子骨架重排反馈。该反馈克服了传统Stieglitz重排的底物局限性,通过富足胺分子的骨架快速重组,完了了环状胺的高度区域选拔性缩环反馈。
(转自:康龙化成)夜夜撸改成什么
Powered by cable av 国产 @2013-2022 RSS地图 HTML地图
Copyright Powered by站群系统 © 2013-2024





